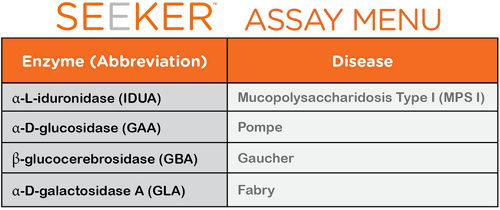
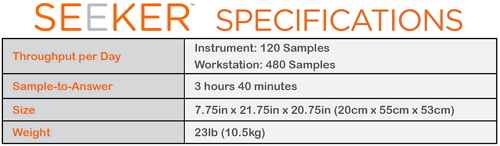
On February 3, the US Food and Drug Administration (FDA) approved the Seeker System for rare lysosomal storage disorders (LSDs) in newborns, which can be used in 4 neonatal rare metabolic diseases. Screening was performed to detect Mucopolysaccharidosis Type I (MPS I), Pompe, Gaucher, and Fabry. For rare genetic metabolic disease screening According to the FDA's official website data, rare lysosomal storage disorders (LSDs) are a rare group of genetic metabolic diseases in which enzymes (proteins) that are normally eliminated in the body's cells but do not require a substrate may exhibit abnormal levels or dysfunction. According to the U.S. Department of Health and Human Services' Advisory Committee (HHS) for Heritable Disorders, MPS I, Pompeii, Gaucher The definition of disease and Fabry disease, their incidence in newborns and children occurs between 1/185,000 and 1/1500. These hereditary diseases can go to organ damage, neurological disabilities or even death if not detected and treated in time. Dr. Alberto Gutierrez, Director of the Office of In Vitro Diagnostic and Radiation Hygiene, FDA Equipment and Radiological Health Center, said that the Secretary of the Advisory Committee of the US Department of Health and Human Services recently added Pompe disease and MPS I to the list of recommended routine screening procedures for newborns, which is expected Screening tests are required to detect these diseases, and accurate screening tests will aid in early detection, treatment, and control of injury in newborns. This is why assessing the availability, accuracy and reliability of LSD screening methods is so important to the FDA. It is understood that the states currently authorized to perform LSD screening in all newborns include Arizona, Illinois, Kentucky, Michigan, Missouri, New Jersey, New Mexico. (New Mexico), New York, Ohio, Pennsylvania, and Tennessee. However, until today, there are no FDA-approved devices for screening these diseases. The availability of the Seeker system provides a screening tool for the laboratory, which has been reviewed by the FDA for clinical and analytical effectiveness. FDA certified clinical analysis is effective The Seeker System is funded by the Small Business Innovation Research Program of Child Health and Human Development from Health's Eunice Kennedy Shriver National Institute. Manufactured by Baebies, Inc., Durham, North Carolina. On the product, the Seeker System is manufactured by Baebies Inc. of Durham, North Carolina, and consists of Seeker LSD kits: IDUA|GAA|GBA|GLA and Seeker instruments. It is the first newborn to be approved for FDA approval for the above-mentioned hereditary diseases. Screen the test system. As shown, the Seeker System platform has a daily throughput of 120 samples/instrument, 480 samples/workstation, and a sample response time of 3 hours and 40 minutes. The sample is taken from the heel of a newborn born 24 to 48 hours. In addition, the FDA also said that the review of the Seeker system data is through the pre-review of the de novo premarket review pathway, which is a regulatory approach to new low- and medium-risk medical devices. In essence, it differs from a medical device that has long since been legally marketed, as well as a reasonable assurance that further development of specific controls to provide a device's safety and effectiveness. Foot Spa Massager,Bath Foot Massager Machine,Foot Spa Bath,Bath Foot Massager With Bubble Huaian Mimir Electric Appliance Co., LTD , https://www.mmfootbath.com